Drug Delivery
HIV Prevention Subcutaneous Drug Delivery Device
Challenge
The Bill and Melinda Gates Foundation funded the development of a subcutaneous drug delivery device for implanting long acting medications aimed at preventing diseases including HIV and Malaria.
Outcome
We did several rounds of concept development, testing, and validation with physicians in United States and Kenya. We also vetted numerous CMOs on behalf of the client and are currently working with them to get FDA approval and put the device into production.
These plastic trocars are the current standard of care for delivering subcutaneous payloads. Unfortunately, they are complicated to use and error prone, resulting in partially inserted payloads, payloads placed too deep, and dangerous infections. The image on the right is a placebo payload used for testing.
Clockwise from the top left, one of hundreds of 3D printed prototypes on the printer, assembling prototypes for user research, remote research kits being prepared for shipment to clinics in Kenya, a line up for user research prototypes ready to be packaged and shipped.
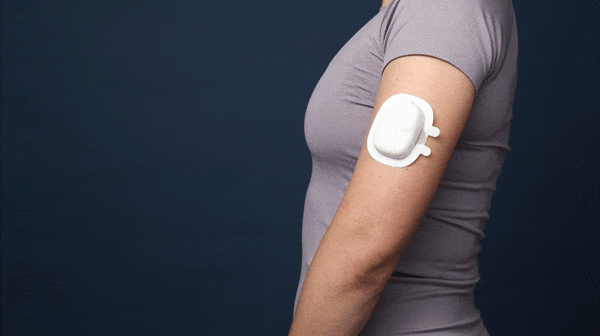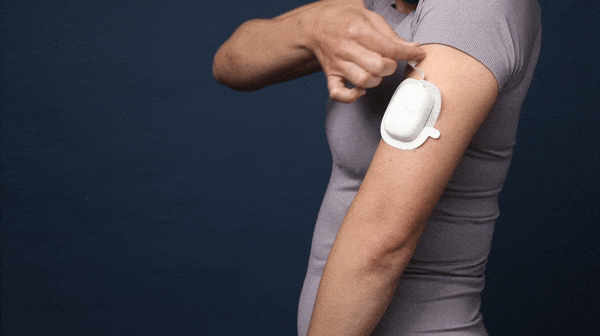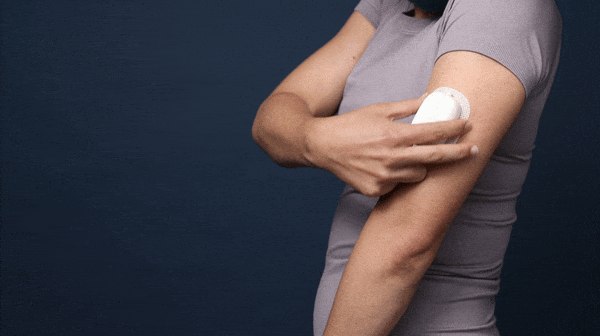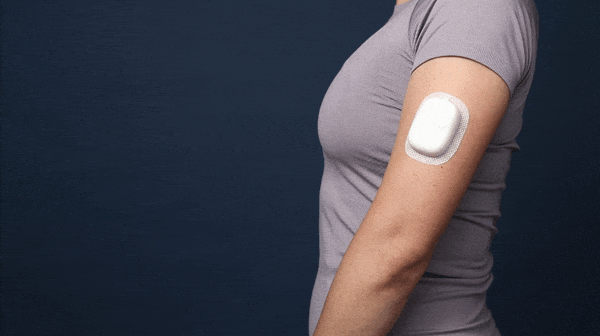
One of the main failure modes that was uncovered during the early research was the risk of premature needle retraction due to physicians placing their fingers on the “needle release button” during needle insertion (Left). We solved this issue by creating a “pull up to release” tab that makes accidental release extremely unlikely.
Here is a small subset of the manufacturing and assembly concepts that we created before landing on the simplest, most robust, and most cost effective design shown below.
This is the final concept. The assembly consists of three injection molded components - the main shell, the needle car, and the back shell. All snap together without the need for glue, ultrasonic welding, or any other processes.
This shows the simple lock out mechanism used to keep the needle retracted and prevent the device from being reused. You can also see the flag on the needle car that serves as an indicator that the device has been retracted.
A demo of the final “works like” + “looks like” model for the delivery device.